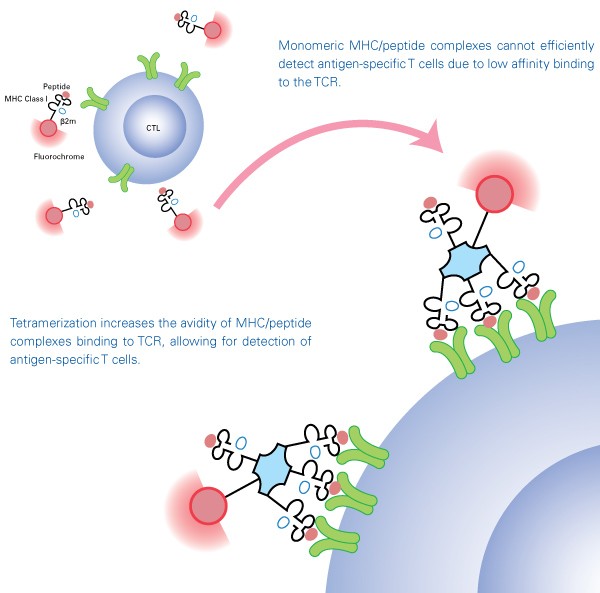
Tetramers
TCRs recognize and bind to complexes composed of MHC molecules and specific peptides expressed on the surface of antigen-presenting cells. While it was once believed that antigen-specific T cells could be detected using soluble MHC/peptide complexes, monomeric MHC/peptide complexes proved to be impractical due to their instability and low affinity to the TCR. To overcome this difficulty, MHC/peptide monomers are biotinylated and tetramerized with streptavidin to maintain stable binding to multiple TCR, enabling MHC/peptide tetramers to be used as detection tools. MHC tetramers are labeled with fluorescent molecules including phycoerythrin (PE), allophycocyanin (APC), or Brilliant Violet™ 421 (BV421) and thus allow detection of antigen-specific T cells by flow cytometry or fluorescence microscopy.
Greater specificity: The patented α3 mutation
The human leukocyte antigen (HLA) system is the name of the major histocompatibility complex in humans. CD8 molecules are known to assist binding of HLA to CTL in vivo and thus HLA molecules have binding sites for CD8 molecules. Bodinier et al. reported that introducing a mutation (A245V) in the HLA class I heavy chain α3 domain minimized unwanted binding to CD8 molecules and dramatically improved specificity (Nat. Med. 2000, 6: 707). MBL has incorporated the patented mutation of the α3 domain in their HLA class I tetramers and is the only manufacturer of MHC multimers with this technology to enhance specificity for the antigen-specific T cell receptor.