Powder: -20°C for 3 years | In solvent: -80°C for 1 year
Etoposide [33419-42-0]
Cat# T0132-100mg
Size : 100mg
Brand : TargetMol
Etoposide
Catalog No. T0132 CAS 33419-42-0
Synonyms: VP-16, VP-16-213
Etoposide (VP-16-213) is a topoisomerase II inhibitor that inhibits DNA synthesis by forming a complex with topoisomerase II and DNA (IC50=60.3 μM). Etoposide has antitumor activity and induces apoptosis and autophagy.
All products from TargetMol are for Research Use Only. Not for Human or Veterinary or Therapeutic Use.
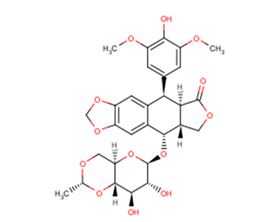
Etoposide, CAS 33419-42-0
| Description | Etoposide (VP-16-213) is a topoisomerase II inhibitor that inhibits DNA synthesis by forming a complex with topoisomerase II and DNA (IC50=60.3 μM). Etoposide has antitumor activity and induces apoptosis and autophagy. |
| Targets&IC50 | Topo II:60.3 μM |
| In vitro | METHODS: Human cervical cancer cells HeLa were treated with Etoposide (25-400 μM) for 24-48 h, and cell viability was measured by MTT. RESULTS: Etoposide inhibited the proliferation of Hela cells with IC50s of 167.3 μM and 52.7 μM for 24 h and 48 h, respectively. The IC50 of Etoposide was 167.3 μM and 52.7 μM at 24 h and 48 h, respectively. [1] METHODS: Human lung adenocarcinoma cells A549 were treated with Etoposide (0.75-3 μM) for 4 h. The cell cycle was detected by Flow Cytometry. RESULTS: Etoposide caused a significant decrease in the percentage of A549 cells in G0/G1 and S phases. Meanwhile, the percentage of A549 cells in G2/M phase was significantly increased. [2] METHODS: Mouse embryonic fibroblast MEFs were treated with Etoposide (1.5-150 μM) for 3-18 h, and the expression levels of target proteins were detected by Western Blot. RESULTS: 150 μM Etoposide induced a strong cleavage of caspase-3 within 6 h, while 1.5 or 15 μM activated caspase-3 only after 18 h. [3] |
| In vivo | METHODS: To detect anti-tumor activity in vivo, Etoposide (10 mg/kg) and Cisplatin (5-7.5 mg/kg) were intraperitoneally injected every two days for two weeks into KSN nude mice harboring human endometrial adenocarcinoma tumors Ishikawa. RESULTS: Etoposide, as a single agent, had little or no inhibitory effect on tumor growth, while the combination of Etoposide and Cisplatin significantly inhibited tumor growth. [4] METHODS: To detect anti-tumor activity in vivo, Etoposide (80 mg/kg in 0.5% methylcellulose) was administered by gavage to immunodeficient mice harboring human glioblastoma tumor U87 once a day for 40 days. RESULTS: 80 mg/kg Etoposide inhibited U87 tumor growth by 95%. [5] |
| Kinase Assay | Nuclear extracts are prepared, and nuclei are isolated. The activity of topoisomerase II is calculated from the percentage of decatenation obtained. Tritiated kinoplast DNA (KDNA 0.22 μg) is used as a substrate. Etoposide and topoisomerase II are incubated for 30 min at 37 ℃ and are stopped with 1% sodium dodecyl sulfate (SDS) and proteinase K (100 μg/mL). The percentages of decatenation and inhibition of topoisomerase II by Etoposide are obtained [5]. |
| Cell Research | After the Etoposide treatment, cells are removed from the dish with phosphate-buffered saline (PBS) containing 0.03% trypsin and 0.27 mM ethylenediaminetetraacetic acid (EDTA) and are diluted into culture dishes in appropriate numbers to yield between 20 and 200 colonies. After 12 days, cultures are fixed with methanol-acetic acid, stained with crystal violet, and scored for colonies containing more than 50 cells [5]. |
| Animal Research | The in vivo model for nude mice HB (NMHB) has been established. Only HB cells with embryonal components are grafted and reproduced successfully in this model. Each NMHB subsequently is transplanted into 50 mice for treatment groups. Treatment is initiated when the majority of the tumors reach a volume of 50-100 mm3. The mice are stratified according to their tumor volume and randomly assigned to groups of ten animals each. The animals injected with tumor are given ifosfamide, cisplatin, doxorubicin, etoposide (10 mg/kg/day, i.v.), and carboplatin as single agents in two blocks. One group of ten animals for each original xenograft served as a control group. After initiation of treatment, the tumor growth is recorded at 5-day intervals for 25-30 days and the relative tumor volumes are calculated. Twenty-four hours before the animals are sacrificed, bromodeoxyuridine (BrdU) is injected intraperitoneally for the semiquantitative determination of proliferation activity of the tumor cells (50 μg of BrdU/g body weight) [4]. |
| Source |
| Synonyms | VP-16, VP-16-213 |
| Molecular Weight | 588.56 |
| Formula | C29H32O13 |
| CAS No. | 33419-42-0 |
Storage
Solubility Information
DMSO: 58.9 mg/mL (100 mM)
 References and Literature
References and Literature
1. HGN ÇELEBİOĞLU, et al. Effects of thymoquinone and etoposide combination on cell viability and genotoxicity in human cervical cancer hela cells. Istanbul J Pharm. 2022; 52(3):258-264. 2. Litwiniec A, et al. Low-dose etoposide-treatment induces endoreplication and cell death accompanied by cytoskeletal alterations in A549 cells: Does the response involve senescence? The possible role of vimentin. Cancer Cell Int. 2013 Feb 5;13(1):9. 3. Jamil S, et al. Etoposide induces cell death via mitochondrial-dependent actions of p5Cancer Cell Int. 2015 Aug 7;15:79. 4. Suzuki M, et al. Anticancer activity of the combination of cisplatin and etoposide in endometrial cancer-bearing nude mice. Gynecol Oncol. 1991 Apr;41(1):41-5. 5. Panigrahy D, et al. Inhibition of tumor angiogenesis by oral etoposide. Exp Ther Med. 2010 Sep;1(5):739-746. 6. Lee KI, et al. Etoposide induces pancreatic β-cells cytotoxicity via the JNK/ERK/GSK-3 signaling-mediated mitochondria-dependent apoptosis pathway. Toxicol In Vitro. 2016 Jul 26. pii: S0887-2333(16)30147-3. 7. Calvani M, det al. Etoposide-Bevacizumab a new strategy against human melanoma cells expressing stem-like traits. Oncotarget. 2016 Jun 9. doi: 10.18632/oncotarget.9939. 8. Zhang J, Hirst A J, Duan F, et al. Darren Robinson, 3 Mark Jones, 2 Le Li, 4 Peizhe Wang, Peng Jiang, 4 Peter W. Andrews, 2 Ivana Barbaric, 2,* and Jie Na[J]. Anti-apoptotic Mutations Desensitize Human Pluripotent Stem Cells to Mitotic Stress and Enable Aneuploid Cell Survival. Stem Cell Reports. 9. Ruan C, Wang C, Gong X, et al. An integrative multi-omics approach uncovers the regulatory role of CDK7 and CDK4 in autophagy activation induced by silica nanoparticles[J]. Autophagy. 2020. 10. Weizhe Li, Hong-Yan Wang, Xiaolu Zhao, Hongguo Duan, Binghua Cheng, Yafei Liu, Mengjie Zhao et al. A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair [J]. Science Advances. 2019 Mar 6;5(3):eaau7566.
 Citations
Citations
1. Yang C, Xu H, Yang D, et al.A renal YY1-KIM1-DR5 axis regulates the progression of acute kidney injury.Nature Communications.2023, 14(1): 4261. 2. Qu Y Q, Song L L, Xu S W, et al.Pomiferin targets SERCA, mTOR, and P-gp to induce autophagic cell death in apoptosis-resistant cancer cells, and reverses the MDR phenotype in cisplatin-resistant tumors in vivo.Pharmacological Research.2023: 106769. 3. Wang D, Wang Y, Di X, et al.Cortical tension drug screen links mitotic spindle integrity to Rho pathway.Current Biology.2023 4. Ruan C, Wang C, Gong X, et al. An integrative multi-omics approach uncovers the regulatory role of CDK7 and CDK4 in autophagy activation induced by silica nanoparticles. Autophagy. 2020 5. Yang G, Wan P, Xiang Q, et al. E3 Ubiquitin Ligase ASB17 Promotes Apoptosis by Ubiquitylating and Degrading BCLW and MCL1. Biology-Basel. 2021, 10(3): 234. 6. Feng J, Xi Z, Jiang X, et al. Saikosaponin a enhances Docetaxel efficacy by selectively inducing death of dormant prostate cancer cells through excessive autophagy. Cancer Letters. 2022: 216011. 7. Li W, Wang H Y, Zhao X, et al. A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair. Science Advances. 2019, 5(3): eaau7566 8. Zhang J, Hirst A J, Duan F, et al. Darren Robinson, 3 Mark Jones, 2 Le Li, 4 Peizhe Wang, Peng Jiang, 4 Peter W. Andrews, 2 Ivana Barbaric, 2,* and Jie Na[J]. Anti-apoptotic Mutations Desensitize Human Pluripotent Stem Cells to Mitotic Stress and Enable Aneuploid Cell Survival. Stem Cell Reports. 2019, 12(3): 557-571 9. Kong Y, Liu Y, Li X, et al.Palmitoylation landscapes across human cancers reveal a role of palmitoylation in tumorigenesis.Journal of Translational Medicine.2023, 21(1): 1-19. 10. Wu X, Yi X, Zhao B, et al.The volume regulated anion channel VRAC regulates NLRP3 inflammasome by modulating itaconate efflux and mitochondria function.Pharmacological Research.2023: 107016.



